Four years later..... a new pandemic approaches.
 (Photo Credit Boston Globe)
(Photo Credit Boston Globe)
It has been four years exactly -- to the day -- that I wrote my last blog. That blog was the COVID-19 Mexican Standoff, and if you read it, I think it holds up very well. I stopped blogging because so much was happening so quickly, it was difficult to stay abreast of developments. The conventional and social media press took over the narrative, and I decided to drop back and let the infectious disease experts take over. In other words, I got out of the way.
Now, four years later, I am rebranding my efforts toward the larger topic of resilience. "Resilience" is a hot word these days, covering everything from pandemics to natural disasters to more human-on-human endeavors.
But my top concern is pandemics. It is what I am best known for, and I will not disappoint. And I see two potential pandemics on the horizon: H5N1 Bird Flu, and Chronic Wasting Disease. Mike Osterhoilm warned us in 2020, on Joe Rogan's podcast, that this disease would jump species, and it has.
But the more immediate threat is our old nemesis avian influenza. Bird flu virus in milk: Sample bought in Mass. tests positive (bostonglobe.com) and Scientists call new measures to control bird flu in cows ‘a drop in the bucket’ | Science | AAAS detail how inadequate and disjointed our countermeasures are at this time.
Longtime readers of this blog understand the mechanics of the imfluenza virus, and you would be rightly concerned about the 50% death rate for cats drinking raw milk from infected cows. You would also be rightly concerned about the mentions of pigs. Pigs, as you (should) know, are the "mixing vessel," able to be infected by bird flu and human flu simultaneously. This "mixing vessel" can the ninternally cook up an avian flu that contains just enough human segments to produce a pandemic strain of a killer flu never before seen.
You would also be rightly scared to read this new "what doesn't kill you makes you stronger" mentality behind the explosive growth of raw milk product consumption. I can see a day approaching when raw milk products are banned from store shelves and from sale at dairies.
So, it is good to be back, because I do have things to say, and I will most definitely say them. See you soon.
Scott
The COVID-19 Mexican Standoff
Everyone is asking the same question: Why have we not been overwhelmed, coast to coast, by this novel coronavirus? It is a legitimate question. Some say it is because the virus is an underachiever. That the lockdowns, isolation and closure of everything was an overreaction. Much ado about nothing.
That is not an accurate depiction of what has happened, however. Here's what really happened: Due to our swift and thorough actions, we have the virus in a COVID-19 Mexican Standoff. And the virus has us in the same position.
There are several reasons why the COVID-19 Mexican Standoff has occurred. First was the rapid sequencing of the coronavirus’ genome. In January of this year, it took Chinese researchers less than a month to sequence the genome of that we now call SARS-CoV-2, the virus that produces COVID-19 illness. Compare that to the original SARS virus in 2002-03, when it took some twenty weeks to sequence. The dizzying speed with which this new SARS-CoV-2 virus’ sequence was then digitally transmitted to PCR testing machines all over the world was a marvel of collaboration and a testament of just how far we have come with medical technology in seventeen years. Testing began at a rate never before seen globally.
At the same time, the Internet -- light years ahead of where it was in 2003 -- became an information (and disinformation) hub. People were able to obtain all kinds of information rapidly. The 24-hour news cycle pumped the images of suffering and death from all over the world, onto televisions and devices across America. Social media became the filter for commentary for a lot of this information. China -- no, Italy! -- became the focus of the world's attention.
Then the cases started in the United States. Seattle, with its world-class public health infrastructure, endured what we thought were the first cases. Then our attention turned to New York City. Then Boston. Then back again to the West Coast, and San Francisco.
Based on this deluge of information, and armed with the historical accounts of what the nation and their communities went through in 1918, political leaders listened to the health care professionals advising them. They almost universally reacted en masse, and the same way. They closed their cities and states. They closed America. But even before the political leaders started formulating policy and making decisions, the American people did their own calculus and decided the risks of traveling and venturing outside of their homes was too great. Nowhere was this more apparent than in sunny Florida, the third largest state in the Union, a state with the highest population of the elderly, numerically and per capita. A state that, by all rights, should have been decimated by the virus.
Except it wasn't. Or, more accurately, hasn't been decimated yet. The Tampa Bay Times story I have linked to is a commendable narrative on just how quickly Floridians made their independent decisions to stay home and not go outside. Of course, Florida still has been hit, but not nearly as bad as the Northeast.
What was the effect of all this? The interventions government implemented – social distancing, closing schools and business and government and shopping and dining and exercising and congregating – all those interventions wound up actually beating the virus to Main Street. The effort was hugely successful! The virus did eventually arrive, but largely found fewer human hosts than expected, based on this universal adoption of these inventions. This lowered the dreaded R-naught (R0) to a figure so low, the virus had great difficulty spreading.
If you read the interviews with the architects of Social Distancing as national pandemic policy during the George W. Bush Administration, whey will all tell you they never thought it would work to the degree it did. As Dr. Howard Markel, author of “When Germs Travel” and one of the architects of the “W” Social Distancing policy, recently told the New York Times:
Dr. Markel called it “very gratifying to see our work used to help save lives.” But, he added, “it is also horrifying.”
“We always knew this would be applied in worst-case scenarios,” he said. “Even when you are working on dystopian concepts, you always hope it will never be used.”
The exceptions to this narrative are painful. New York City. Boston. Dougherty County (Albany), Georgia. Nursing homes everywhere. Meat packing and processing houses nationwide. Situations as strange and varied as the virus is unpredictable. But for most of America, the virus has not yet been allowed to gain a foothold. And nowhere has the virus been allowed to reach fully into the country. Not yet. My friend and mentor Dr. Michael Osterholm told USA Today this week:
"This damn virus is going to keep going until it infects everybody it possibly can," Osterholm said Monday during a meeting with the USA TODAY Editorial Board. "It surely won’t slow down until it hits 60 to 70%" of the population, the number that would create herd immunity and halt the spread of the virus.
"It’s the big peak that’s really going to do us in," he said. "As much pain, suffering, death and economic disruption we’ve had, there’s been 5 to 20% of the people infected, ... That’s a long ways to get to 60 to 70%."
Osterholm acknowledges that the nation "can't lock down for 18 months" and said political and business leaders need to find a way to resume activities while adapting to a virus that won't soon disappear. He doesn't believe there has been enough of a frank assessment on the economic harm the virus will cause over coming months and its disruption to international supply chains.
"We all have to confront the fact there’s not a magic bullet, short of a vaccine, that’s going to make this go away," he said. "We’re going to be living with it. And we’re not having that discussion at all."
So let’s have that discussion. In order to have it, we need to accept that as we outraced the virus, we also sailed into Uncharted Waters. In other words, we have never been here before. This is the first pandemic in history where the response outpaced the spread of the virus - hence our Mexican Standoff.
So to move forward, the Mexican Standoff must end. It ends by us making some decisions. We need to make the decision to move forward, selectively reopening America, and looking to see what the virus’s next move is. We must be patient but we also must not divert from this strategy. Until we have an effective vaccine that the virus cannot mutate itself around, we must carefully maneuver toward at least partial herd immunity via exposure. But we need to be careful and not be reckless.
So what decisions are to be made, exactly? Because we have never been here before in human history, we can say that there are no bad decisions, until we start generating data from all these reopenings and see what worked and what may have put Americans under unacceptable risk. As Mike Osterholm said, we cannot stay closed for 18 months. In my opinion, probably not even 18 more weeks. We cannot keep schools, colleges and universities closed indefinitely. Our way of life is at risk.
Many point to Sweden as the global best practice for forcing Herd Immunity upon its citizens. We can look at Sweden, but we should also be looking closely at Georgia, and Florida, and every other state which is trying to navigate those Uncharted Waters. The jury is still out on Sweden. Their strategy is not a done deal. They cannot declare success just yet.
We know that as we reopen, case numbers are going to go up. That is inevitable. Maybe the summer heat and humidity will work on the virus. We simply so not know if summer will make the virus wilt or not. We need to be prepared for this scenario not to happen. But if it does, then summer heat and humidity become our allies and we need to be ready to exploit them.
We need to redouble our efforts to identify and protect those with comorbidities and protect the vulnerable among us. Identify those most at risk from the virus and have a strong plan to keep them out of harm’s way. Keep these groups working from home if necessary. Do our part as neighbors and minimize their trips to the market. Toward this, government data is perhaps the most important commodity. Is VISTA still around?
Despite the CDC’s recent reversals, we still need to continue to clean solid surfaces. Routinely and maniacally. At work, at home, at businesses, at the grocery store, at theatres and concert venues. Do this for morale if nothing else. I am still betting solid surfaces transmit COVID.
Open the schools. Kids for the most part are not getting seriously ill, and if we are going to reopen the nation, parents need to be able to send their kids to school. You will not have a substantive economic rebound unless and until schools reopen. What is the best way to achieve social distancing in schools? Here's an idea: When I was at Pompano Beach Junior High School, I had to endure the Hell of Double Sessions. And I lived to tell the tale! Split the sessions. Separate the students. Pay the teachers for the extra work, or hire some additional staff, or have classes in the cafeteria, or the auditorium, or the gym, and spread the kids out and combine classes. But you've got to get the kids back in the classrooms if you want economic recovery. And by the way, make sure the football players are in the early schedule. We need football!
Kids and teachers can wear masks. Kids will wear them so they do not infect their teachers. And vice versa. Kids might not hear their teacher well through the mask, so here’s a novel concept: Listen closely!
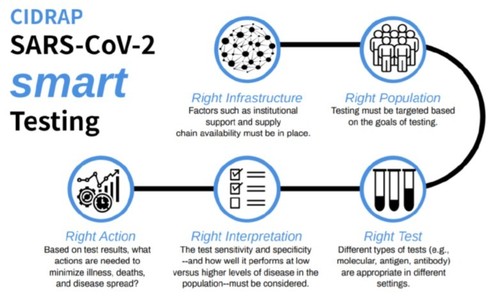
We need to completely rethink our testing strategy. As Mike Osterholm said yesterday, "It's a mess out there." The CIDRAP report on testing was released yesterday and it bears analysis. It does push for national standards and involvement, but it also makes the point I am making here: We need a reliable supply chain for these tests, as the virus returns in the fall and as the virus continues to pop up during the summer. I have proposed stockpiling tests – including antibody tests – for the anticipated Second Wave of the virus, sometime in the fall. However, we equally need to test now to confirm when we see the spikes coming from reopening. We have got to ensure we have enough tests for the second wave, and a stable supply chain to provide them. Couple this with some world-class contact tracing, for which we need to be doing our building and training now for the fall. If we stockpile enough tests now, we should have more than enough to fully engage in the Second Wave. Plus, we should have remdesivir, hopefully in enough quantities to administer it to the highest-risk groups and first responders early in their infections, when it will decidedly do the most good.
If we see a spike in cases, and if we are blocking and tackling as well as we should be, then we should be able to respond to the spike and bring it down without having to resort to the same harsh blanket responses as we used in the beginning of this pandemic.
And we need to wear masks in public. The purpose of wearing masks is not to avoid getting sick. It is about keeping your germs to yourself. Wearing a mask greatly reduces the chance you will infect someone else. Refusing to wear masks to make a political point is not the time to be making a political point. Wearing masks is not a Constitutional issue, and thus, refusing to wear masks is not a God-given right, according to Alan Dershowitz. He cites the Tenth Amendment. Anyone here want to debate him on Constitutional grounds? Are we really serious about wrestling this virus into submission? You want everything open? Wear a mask. Please.
If you are watching Sweden (3,832 fatalities as of this blog) and (loudly) pointing to their herd immunity program, then to be fair, you must also point to Japan (768 fatalities nationwide) and trumpet Japan’s ability to keep their fatalities well under Sweden’s, simply by wearing masks. Combining Sweden’s herd immunity strategy with Japan’s mask adoption will see us through this crisis, until we have either a vaccine, or inhalable remdesivir, or both.
NOTE: This blog was edited following the release of the CIDRAP testing guidance.
Latest news on the drug front against COVID-19
I just had an old friend (he is youthful, we've just known each other for 21 years) reach out to me about the status of drug companies' efforts to beat back COVID-19.
I had not peeked in recently on that front, so I thought it would be appropriate.
So here we go:
There are vaccine candidates but the time from trials to an actual vaccine will seem to take forever. A year to eighteen months is the Best Guess. While people scoff at that timeframe, the reason is quite simple: They do not want to inject anyone with something that might be worse than the disease. Did they not read I Am Legend? Anyone ever hear of thalidomide? Google it if you are unfamiliar with the drug and its side effects. Have tissues ready.
The experiments that treated coronavirus patients with existing HIV treatments have all apparently failed.
https://www.nytimes.com/2020/03/18/health/coronavirus-antiviral-drugs-fail.html
Breathless headlines suddenly are touting a malaria treatment as a possible remedy for coronavirus. Ditto a known blood pressure drug. A 1500-person study is going on now.
Persons with hypertension appear to be at a much greater risk of serious complications from COVID, so it will be interesting to see any correlation.
The Japanese have an antiviral called favipiravir, and this article in The Guardian says it is promising. But it is The Guardian, so you take it with a grain of salt. It also appears that like Tamiflu, the window is very short before the drug loses all effectiveness.
So science and medicine are throwing every antiviral they have in the medicine cabinet against this virus. I could pull out the COVID diagram and show you the S-protein and show you how and where it enters a cell (the "cleavage site"), but then your eyes would glaze over and I would lose you. I would lose me, too.
And then there's remdesivir. The feds are in trials now, a whopping 453 people (satirically said). The trials are due for an initial conclusion next month. Final conclusion in May. Gilead, the inventor/patentholder, is doing a 1,000 person trial in Asia. Should've been Italy, IMHO. If remdesivir appears to work, expect to see Gilead stock leading a huge buying surge on Wall Street. My uninformed stock prognostication. Interesting the front-end load is 200mg, then daily 100mg. Exactly what I do with Tamiflu: Two capsules to start, then a capsule twice a day.
https://clinicaltrials.gov/ct2/show/NCT04257656
https://www.biospace.com/article/gilead-launches-2-phase-iii-trials-of-remdesivir-for-covid-19/
BREAKING NEWS! CIDRAP (the Center for Infectious Disease Research and Policy at the University of Minnesota, aka Mike Osterholm's organization) has just released a very detailed look at the topic I just blogged about. CIDRAP's article has great detail about a brand-new initiative the WHO is doing, called the SOLIDARITY trial. Please take the time to read it. They just retweeted this very blog entry as well, so a hat-tip to Mike and the team at CIDRAP!
And if anyone sends you that Internet crap about gargling curing the virus: flog them.
Will warmer temperatures bring a respite from COVID-19?
As of this writing, the WHO has finally proclaimed COVID-19 a true pandemic. Anticlimactic to be sure, but final and now the P word has been uttered and we can get on with things.
The number of coronavirus cases in the United States has topped 4,660 as of this writing. It was 1,300 when I began drafting this post a few days ago. Meaning, we are just seeing the tip of the tip of the iceberg. As we test, we find more cases. This is as inevitable as the tides. We still do not know what the virus will ultimately look like and how it will behave. It is trending toward the informed theory, so far: roughly 8 in ten cases will be relatively mild; 2 in ten will be problematic, with a significant percentage of those cases headed straight to the ICU. People ages 55 and older are the principal worry, with the most elderly and frail among us in frightful danger if they catch it. Children are treated (so far) lightly by the virus, but speculation, led by former CDC director Thomas Frieden, is because these children are asymptomatic carriers of the virus itself. Sobering yet unproven thought.
Cases outside of China are giving us much more useful data. Sputum harvested from German and Italian sufferers indicates that while "recovered" patients may still test positive, they are not contagious. Scientists are hovering around Day 8 as the day they stop being contagious.
Unfortunately, we are now seeing clusters turning to outbreaks in diverse areas of the United States. The Greater Seattle area and New Rochelle, New York, are battling it out for numerical supremacy. The approaches to combating the virus are as different as the two metropolitan areas. Seattle, and King County as a whole, are using their world-class public health system and implementing strategies the Chinese deployed (minus dragging screaming people out of their homes at gunpoint).
But the Washingtonians are facing more draconian measures. A few days ago, the government of King County called upon all citizens to work from home, and asked all citizens ages 60 and older to stay in their homes. Now, just this morning, the Governor of Washington State has shut down all bars, restaurants and recreational facilities.
New York, on the other hand, has called out the National Guard and drawn a one-mile "containment zone" around the epicenter of their outbreak, a synagogue. They have banned public gatherings. The Guard will be used to decontaminate public buildings and public areas, deliver food to shut-ins, and assist in logistics. I would have to assume they will also be filling in for essential government functions if absenteeism soars.
The tri-state area that comprises New Jersey, New York and Connecticut are acting jointly to ban all public gatherings, period. The lights are out on Broadway. Bars and restaurants are open for take-out only. Gyms and fitness centers are shuttered. Schools closed, probably for at least a month.
None of this is about the heat, so far. I just wanted you to get up to date.
Now the heat. The Conventional Wisdom is that viruses hate the heat. The moisture in the air, coupled with warmer temperatures, should literally knock the virus down. Same theory as a hit baseball carries farther in Denver than it does in Atlanta. Heat and humidity disrupt the ball's velocity, etc.
But that does not always ring true. Almost every flu pandemic over the past 300 years has started outside of winter. And every virologist worth his or her salt is hedging bets, saying forthrightly and truthfully that we don't know anything about the resiliency of this virus.
So here are some articles to consider. First is a brand-new article from Business Insider. In it, the publication draws upon the news of the recent infection of Tom Hanks and his wife as he was filming an Elvis movie (he plays Col. Tom Parker). They were filming on Australia's Gold Coast, where the weather is a balmy 75 degrees Fahrenheit. Salt air and everything.
Then you look at the map of the world's COVID-19 cases, compiled so brilliantly by Johns Hopkins University. Look at all the cases in the Southern Hemisphere. These cases are growing as the world's cases are growing.
Last week, there was an excellent article in National Geographic, titled "Will warming temperatures slow the coronavirus outbreak?" There is an abundance of evidence to support the belief. People won't cluster together in hiding from the elements. School will be out (at least hypothetically. No one has yet, to my knowledge, posited the theory that the school year might be lengthened into the summer due to school closures, so I will posit that right now). And the virus will drop harmlessly to the ground, inert, to be mopped or vacuumed into obscurity.
But the opposing side also has compelling arguments. Theses are also based on scientific historical research. Newbie viruses ignore the conventional wisdom. They laugh at increased humidity and higher temperatures. They play dead and subside, until the weather changes and they come roaring back to life. The 1918 and 2009 pandemics are instructive. The viruses in both pandemics appeared to disappear in late spring, only to come roaring back at the first sign of crisper air, more lethal and more infectious than ever. Lethal and 2009 are not usually seen, but there are numerous cases of children succumbing to the virus. So be very careful when you say the 2009 virus was not a killer. Someone within earshot might vociferously disagree. And another reason is that the virus has not burned through all its available hosts. That is precisely why we are socially distancing all over the place. Deny the virus a host and it will go away. Flatten the curve!
Another version of the same theories can be found here.
But by far, the most instructive analysis of the topic can be found at the blogsite of Marc Lipsitch, DPhil
Professor of Epidemiology and Director, Center for Communicable Disease Dynamics, Harvard T.H. Chan School of Public Health. Dr. Lipsitch is a certified disease rock star and is very quotable. His short answer to the question:
Will COVID-19 go away on its own in warmer weather?
His answer:
"Probably not."
He then proceeds to say why he doesn't believe it will not go away. From his essay:
For the novel coronavirus SARS-CoV-2, we have reason to expect that like other betacoronaviruses, it may transmit somewhat more efficiently in winter than summer, though we don’t know the mechanism(s) responsible. The size of the change is expected to be modest, and not enough to stop transmission on its own. Based on the analogy of pandemic flu, we expect that SARS-CoV-2, as a virus new to humans, will face less immunity and thus transmit more readily even outside of the winter season. Changing seasons and school vacation may help, but are unlikely to stop transmission. Urgent for effective policy is to determine if children are important transmitters, in which case school closures may help slow transmission, or not, in which case resources would be wasted in such closures.
So don't bet the farm on the virus taking a break during the summer. And if it does, history says it probably will be back at the first sign of autumn, and it will be upset it was woken up.
How to build instant surge capacity
The lessons first came out of China: In nine days, a 1,000 bed COVID-19 hospital was erected, furnished and staffed. Then sports arenas, civic centers and other large buildings were taken over as makeshift hospitals.
The Chinese philosophy was simple: Keep suspected coronavirus patients separate and far apart from the general population of a hospital. It was a lesson learned hard during the SARS epidemic/almost a pandemic in 2003.
Shift to King County, Washington. King County has long been hailed as the national model for pandemic preparedness. Over the years I have watched numerous press conferences, Webinars and Powerpoints that came from the King County health department. I hold these people in very high esteem. So when a cluster/outbreak began to occur there, I said that was the best possible place in America for that to happen. If it happened anywhere, at least it happened there.
Following the lead of the Chinese government, and following the WHO press conference of a couple of weeks ago when Dr. Bruce Aylward spoke glowingly of how the Chinese adopted that strategy, King County is moving to build surge capacity. Toward that end, King County just purchased a 24-room hotel -- with individual air conditioners, as opposed to central air and heat, which killed so many people in China and Toronto in 2003. Individual A/C units was a key to the purchase. King County plans to send all non-severe COVID-19 cases to that facility, and others like it, if need be.
Ever since COVID-19 got its name, I have ben thinking along similar lines. What facilities could be pressed into service in case this virus takes a foothold and gets people sick? Here in Tallahassee, we have a vacant Kohl's on the northeast side near Chiles High School that would function nicely. All communities have vacant commercial buildings that could be pressed into service quickly. Decision-makers need to scout these locations now and have plans in place to lease or buy them outright, now rather than later. Follow the King County example. That is what at least part of that $8 billion Congress just agreed to should be used for. If this thing turns nasty, that will not be the sole method of building surge capacity. But plans should be made now to implement this strategy.
Another sad fact of life is the (growing) number of hospitals -- mostly rural, but not always -- that are closing. These hospitals just cannot afford to remain in business, for a variety of reasons but largely centering on reimbursement rates versus the costs of care. Each state should compile an inventory of these shuttered hospitals with the intent to re-open them as COVID treatment centers. We'll need all the beds we can muster, again if this virus takes a nasty turn and starts appearing everywhere.
