Study SARS response and dust off those old pandemic plans
For those of us in the USA, the SARS almost-a-pandemic was just another headline. In Canada, especially Toronto, it became a nightmare. And in Asia, it became a matter of life-and-death. SARS came within a gnat's eyelash of becoming a real pandemic. Remember your Pandemic 101: A pandemic exists when a novel disease that has not previously been seen in humans emerges; it is easily transmissible from person to person; it is truly global geographically; and no or almost no natural immunity exists in the population.
So the only thing that kept SARS from attaining that lofty goal was its inability to replicate itself on every continent and, as a result, to burn through a significant portion of each continent's population. There is an ongoing pandemic -- the HIV/AIDS pandemic - but people usually are not aware that yes, it is a pandemic virus.
Excepting AIDS, there is only one recent playbook on the shelf to fight this: the SARS response. One would do well to Google the hell out of SARS to gain knowledge about coronaviruses in general and how close we came to a real bad situation with SARS, which will help you better understand this new 2019-nCoV.
There is one other playbook on the shelf, or should be: your old, yellowed, dusty flu pandemic plan. What, you say? We have a pandemic plan? Chances are your IT shop has, or had, one. Here in sunny Florida, CIOs were drilled by yours truly to have one until they cried for their mommas. And now it is time for CIOs, CTOs and assorted other IT professionals to find those plans, blow the dust off, refamiliarize themselves with them and vigorously update them.
In 2006, as I was formulating my own pandemic plans and convincing agency heads to do the same, Michael Dell flew in to Tallahassee to address agency IT leaders. During Q and A, I asked Mr. Dell what his company's pandemic plans were. His answer was quite informative. He said they had first-hand experience with this topic during the SARS epidemic. They checked their supply chains for vulnerabilities and deficiencies. They prepared to eventually shift production between Ireland and China based on where the virus was, knowing flu moves in geographic and antigenic waves during a pandemic. But it was the SARS response that would really inform the company's response. It was inspiring. But then Mr. Dell asked me what I thought of his plan, and did I have suggestions?
Well yeah, I thought, but simply told him it appeared he had a good handle on things.
For those of you with pandemic plans, you will probably find the human elaments have not changed much. Will you engage work-at-home plans? What defines WORK, anyway? What are your deliverables in a socially distanced environment? I can tell you we have already addressed these issues in my shop and are preparing to test all ways to access our servers and network and VoIP servers. Can our support staff answer calls from home successfully? Can they work on their laptops and access our help desk software? Can our developers code from home successfully?
Have you adjusted your skills matrix recently? Have you mapped all the tasks an employee performs, and are two to three people able to also perform those tasks? It doesn't mean another person does 100% of those tasks. Quite the opposite: It means you can distribute those tasks across your area so that the work gets done adequately. For flu, we assumed an absentee rate approaching 35-40% at any given time as the virus burns through your community. For this coronavirus, that may be conservative. Or not. We don't know yet. And you don't know who might never come back.
Now for the supply chain. Apple just announced it was implementing plans to deal with supply chain disruptions caued by the pandemic. You should also lump your SaaS and cloud providers into that category. You should be surveying all your key suppliers and asking them what their pandemic plans are. If they cannot articulate their pandemic plans, find alternative suppliers. Ditto your cloud partners. What are their pandemic plans? After all, the cloud is just a whole bunch of data centers, full of people, packed into spaces with sealed HVAC systems.
You need to be asking those questions TOMORROW. You should expect answers the same day. There is no reason to delay doing these things. It is the prudent thing to do.
New coronavirus: Preliminary CFR, incubation period
The only thing that kept the SARS epidemic from going full tilt pandemic in 2003 was its incubation period. SARS was a coronavirus, whose incubation periods are usually 2 to 7 days. People didn't transmit the disease until the incubation period was over. Public health officials were actually able to get in front of the disease and beat it back. Line of sight. Good thing too, because for every 100 people who contracted it, around fifteen died, according to the WHO. That number was impacted by the usual issues of age, general health, and so on. The dreaded 1918 Spanish Flu (H1N1) haad a Case Fatality Rate, or CFR, of 2.5.
Now we don't really know a helluva lot about this new coronavirus, but we suspect it is not as lethal as SARS. At least, not yet. It does currently have a CFR of 3, meaning it appears to be as lethal as the 1918 flu. We are also being treated to conflicting theories as to how transmissible it is (it appears to be quite good at that), and how long the incubation period is.
From The Hill:
"Chinese health officials are warning that the deadly coronavirus could be much more contagious than initially thought, as infected patients can spread the flu-like illness before showing any symptoms.
China’s National Health Commissioner Minister Ma Xiaowei announced during a press conference Sunday the virus is infectious during its incubation period of one to 14 days and its ability to spread is increasing.
The official said authorities have limited knowledge on the new virus, and are unclear on the risks posed by the mutations of the virus.
A longtime adviser to the U.S. Centers for Disease Control and Prevention, Dr. William Schaffner, told CNN the new development means “the infection is much more contagious than we originally thought.”
Schaffner called it a game changer, and warned current preventative methods won’t be enough to fight off the outbreak since tracking down the contacts a patient had before experience symptoms complicates the situation."
The general rule is that viruses get along to go along. They gain nothing by killing their hosts. Their goal is coexistence. But it is also true that as a virus mutates, it can gain some nasty attributes. The second wave of the 1918 flu (fall 1918) was the REAL killer, where most of the 50 to 100 million worldwide deaths occurred.
It is way too early to know what this new coronavirus will mutate into. But we have a virus that is getting easier and easier to catch and transmit, or might have been fron the get-go. And we also have a virus which is not nearly as lethal as SARS or its current cousin MERS (with a CFR nearing 35%). But 3% is what we worried about during bird flu planning assumptions. This concern is real and increasing.
Your morning coronavirus update: January 24, 2020
Wuhan remains under quarantine; twelve other Chinese cities have travel restrictions, bringing the total number of people under restrictions at 35 million, roughly the population of Canada; Disneyland Shanghai to close; more than 800 cases now confirmed in multiple countries, including the US; 26 deaths worldwide, keeping the current Case Fatality Rate at 3%; and a student at Texas A&M may have the disease. He traveled to Wuhan and has flu-like symptoms.
A new weapon in the fight against influenza
The Food and Drug Administration announced yesterday it had granted approval of a new one-dose drug to fight influenza.
It is what this drug does that is so very interesting. Instead of targeting neuraminidase, the substance that allows virus particles to escape from a host cell that has been compromised, this new drug attacks an enzyme that the flu virus needs to replicate itself -- period. Also, instead of a course of capsules, you take one dose and you're treated.
The drug is called Xofluza (baloxavir marboxil) and it is unknown when it will be available in the U.S.
From a September press release:
"Baloxavir marboxil, discovered and developed by Shionogi, has a novel mechanism of action that inhibits cap-dependent endonuclease, an essential enzyme for viral replication. The regimen for baloxavir marboxil is a single-oral dose to treat uncomplicated influenza, which is different from most currently available antiviral treatments. In non-clinical studies, baloxavir marboxil demonstrated an antiviral effect against a wide range of influenza viruses including oseltamivir-resistant strains and avian strains (H7N9, H5N1).12, 13, 14
"Shionogi and the Roche Group which includes Genentech in the U.S. are in a license and collaboration agreement to further develop and commercialize baloxavir marboxil globally. Under the terms of this agreement, the Roche Group holds worldwide rights to baloxavir marboxil excluding Japan and Taiwan where the rights are retained exclusively by Shionogi. Roche will further investigate baloxavir marboxil in a global Phase III development program including pediatric and severely ill hospitalized populations with influenza. Shionogi will conduct a post-exposure Phase III prophylaxis study in Japan in the 2018/2019 flu season."
I do not know if this is the Holy Grail of flu drugs: Something that would attack all strains of the virus and be immune to immunity. But it does appear to be effective against those strains that have acquired an immunity to Tamiflu, and also apparently dramatically reduces the actual viral load of the bug once it is in the human body pretty aggressively. This makes it a much better candidate for combating potential pandemic strains such as H5N1, H5N6, H7N9, and H9N2.
Roche has been manufacturing Xofluza to meet demand in Japan, where the drug went on sale in February. It is unknown when it will be available for sale in the U.S.
2018's flu season surpasses 2009's Swine Flu pandemic, and other takeaways from the latest CDC Flu Report
 The weekly CDC flu report has been released, and it is full of important information. There were several things that jumped out at me, and I wanted to bring them to your attention.
The weekly CDC flu report has been released, and it is full of important information. There were several things that jumped out at me, and I wanted to bring them to your attention.
First: Flu remains everywhere. Oregon seems to have a dip in cases, but I question that. However, 48 other states are positively bathed in flu. It has not abated one whit.
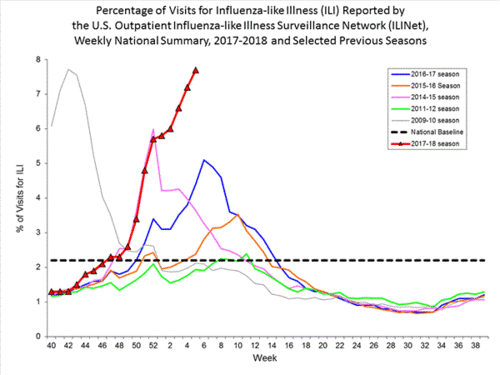 Second, and the CDC has just admitted this: This year's flu epidemic, in terms of hospital visits, has just surpassed 2009's H1N1 swine flu pandemic. Think of that! A non-pandemic strain of flu has sent more Americans to the hospital than the first pandemic in almost fifty years. While of course I am hopeful pediatric deaths will not reach the level seen in 2009, we are seeing pediatric and young adult sickness and death at a rate seldom seen in a flu season.
Second, and the CDC has just admitted this: This year's flu epidemic, in terms of hospital visits, has just surpassed 2009's H1N1 swine flu pandemic. Think of that! A non-pandemic strain of flu has sent more Americans to the hospital than the first pandemic in almost fifty years. While of course I am hopeful pediatric deaths will not reach the level seen in 2009, we are seeing pediatric and young adult sickness and death at a rate seldom seen in a flu season.
Third: Flu season is a marathon, not a sprint. And while the cumulative ratios of A/H3N2 to B has been roughly 80/20 this season, we are seeing a surge in cases of Influenza B. This is nothing to celebrate: As you recall, the young 12-year old boy from West Palm who died, was typed using reverse PCR as having had Influenza B.
Fourth: The strain of B that is the strongest -- the Yamagata strain -- is antigenically very similar to what is in the current flu shot! So despite what you have heard, or read, about this year's weenie performance of the shot, it is still a very, very good idea to have one. Because the B protection is significant, and since B's jockey is showing the horse the whip, it might just continue to gain market share. How many metaphors can I mix here?
Fifth, and this one is a biggie: Laboratory-confirmed influenza-related hospitalizations of persons aged 65 years or older are Off. The. Charts. This acceleration began around Christmas and has not abated one bit. Second place: Persons 50-64. Their ascent is not as dramatic but it is significant.
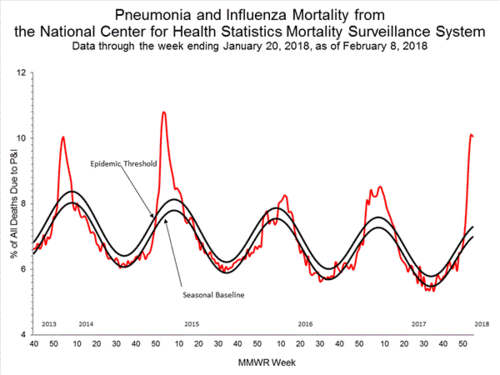
Sixth: Deaths from flu this season are substantially higher than the epidemic threshold. Ten percent of all the deaths in this country were from flu and accompanying pneumonia. The normal epidemic threshhold is just above seven. And because of regional lags in inputting death records, the final figure will be worse.
Seventh: It's amazing what changes happen when you haven't been paying attention. Loyal readers of this blog will instantly recognize the name BioCryst, the pharma company once HQ'ed in Birmingham, Alabama, and which relocated to the Research Triangle of North Carolina. I came across a chart showing antiviral resistance, and listed were three antivirals: Oseltamivir (Tamiflu), zanamivir (Relenza), and -- peramavir! Peramivir is the injectable antiviral created by BioCryst! Apparently they were granted FDA approval back in 2014. I had stopped blogging by then, so it was nice to see that another antiviral had been added to the mix -- and that I was right to keep track of BioCryst.
The complete report is available here. This flu epidemic continues to confound and frustrate.
